Geometries of Transition metal complexes
In this topic, it is important that you remember your study of geometrical shapes (from your maths course).
As examples of what structures transition metal complexes in general can assume, the geometries of Cu(II) water complexes are shown below. Cu(II) means that there is a double charge on the copper atom. Copper complexes are also observed with a single charge, Cu(I), but those present other different geometries (the geometry depends of the charge on the metal, among other factors). The type and quantity of ligands also affect geometry.
Copper water complexes will be used to demonstrate the various geometries assumed by transition metal complexes (bond lengths and angles are shown):
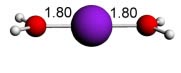
Cu(II) water - 2 ligands - linear
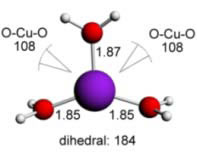
Cu(II) water - 3 ligands - trigonal planar
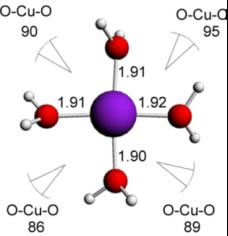
Cu(II) water - 4 ligands - square planar
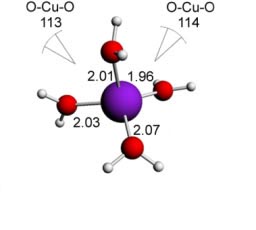
Cu(I) water - 4 ligands - tetrahedral
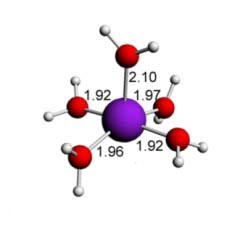
Cu(II) water - 5 ligands - square based pyramid
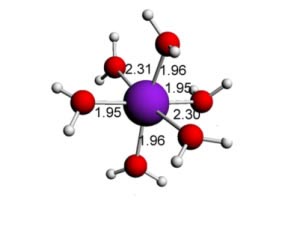
Cu(II) water - 6 ligands - Octahedral (distorted)
more octahedral structures in the ligands page>>
